G Ml To Kg M3
candidatos
Sep 17, 2025 · 5 min read
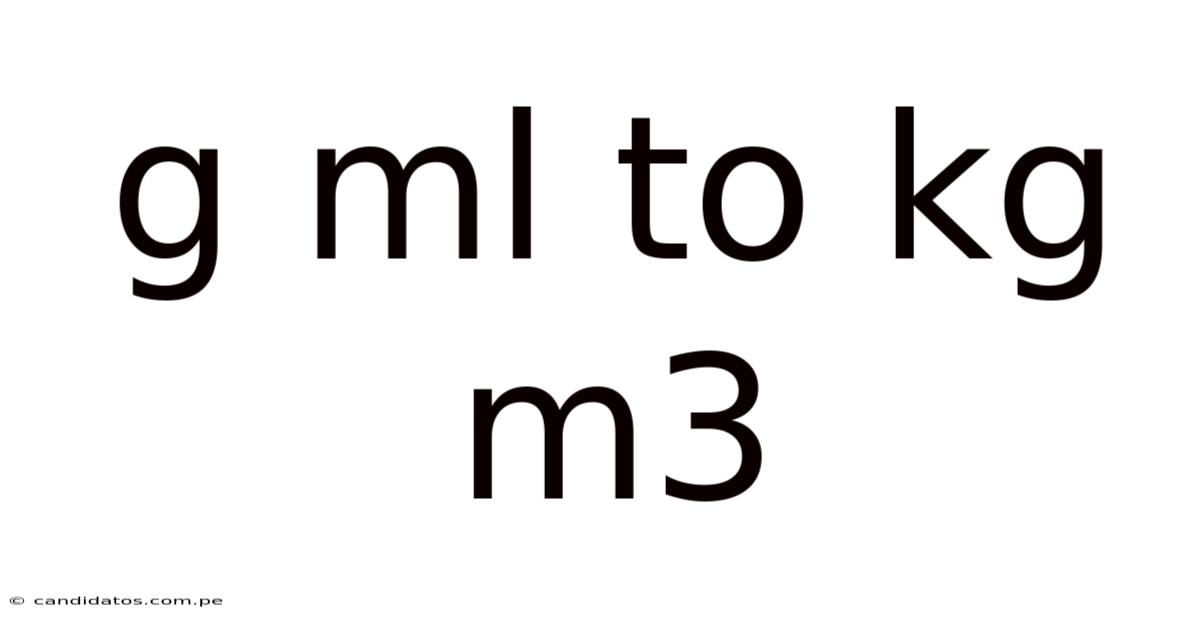
Table of Contents
From g/mL to kg/m³: Mastering Density Conversions
Understanding density is crucial in various scientific fields, from chemistry and physics to engineering and materials science. Density, often expressed as mass per unit volume, allows us to compare how much matter is packed into a given space. This article will guide you through the essential conversion process from grams per milliliter (g/mL) to kilograms per cubic meter (kg/m³), explaining the underlying principles and providing practical examples to solidify your understanding. We'll explore the relationship between these units and delve into the importance of accurate density measurements in various applications. This comprehensive guide will equip you with the knowledge and skills to confidently perform this critical conversion.
Understanding Density and its Units
Density (ρ) is defined as the mass (m) of a substance per unit volume (V): ρ = m/V. The most common units for density are g/mL (grams per milliliter) and kg/m³ (kilograms per cubic meter). While seemingly different, these units represent the same fundamental concept – the amount of matter in a specific volume. The difference lies in the scale and the units used for mass and volume.
-
g/mL: This unit is frequently used in chemistry and often represents the density of liquids. Grams are a relatively small unit of mass, and milliliters are a small unit of volume, making g/mL suitable for smaller-scale measurements.
-
kg/m³: This unit is more commonly used in engineering and physics, especially when dealing with larger volumes and masses. Kilograms are a larger unit of mass, and cubic meters are a larger unit of volume, making kg/m³ appropriate for larger-scale applications.
The Conversion Process: g/mL to kg/m³
The conversion from g/mL to kg/m³ involves converting both the mass and volume units. Let's break down the steps:
-
Mass Conversion: We need to convert grams (g) to kilograms (kg). Since there are 1000 grams in 1 kilogram, we use the conversion factor: 1 kg = 1000 g. Therefore, to convert grams to kilograms, we divide by 1000.
-
Volume Conversion: We need to convert milliliters (mL) to cubic meters (m³). This is a two-step process:
- First, we convert milliliters to liters (L): 1 L = 1000 mL. Therefore, to convert milliliters to liters, we divide by 1000.
- Second, we convert liters to cubic meters: 1 m³ = 1000 L. Therefore, to convert liters to cubic meters, we divide by 1000. Combining these two steps, converting milliliters to cubic meters involves dividing by 1,000,000 (1000 x 1000).
-
Combining the Conversions: To convert density from g/mL to kg/m³, we combine the mass and volume conversions. Since we divide the mass by 1000 and divide the volume by 1,000,000, the overall effect is multiplying the initial density value by 1000.
Therefore, the conversion formula is:
Density (kg/m³) = Density (g/mL) * 1000
Worked Examples
Let's illustrate this with some examples:
Example 1:
The density of water is approximately 1 g/mL. Convert this to kg/m³.
Using the formula:
Density (kg/m³) = 1 g/mL * 1000 = 1000 kg/m³
Example 2:
The density of a certain metal is 7.85 g/mL. Convert this to kg/m³.
Using the formula:
Density (kg/m³) = 7.85 g/mL * 1000 = 7850 kg/m³
Example 3:
A liquid has a density of 0.8 g/mL. Convert this density to kg/m³.
Using the formula:
Density (kg/m³) = 0.8 g/mL * 1000 = 800 kg/m³
Importance of Accurate Density Measurements
Accurate density measurements are crucial in various applications:
-
Material Identification: Density is a characteristic property of a substance; therefore, it can be used to identify unknown materials by comparing measured density to known values.
-
Quality Control: In manufacturing, density measurements ensure consistent product quality. Variations in density might indicate inconsistencies in material composition or processing.
-
Process Optimization: In chemical engineering, density is a critical parameter in designing and optimizing industrial processes like mixing, separation, and transportation of fluids.
-
Environmental Monitoring: Density measurements are utilized in environmental monitoring to determine the concentration of pollutants in water or soil samples.
-
Medical Applications: Density plays a role in medical imaging techniques like X-ray and CT scans, where different tissue types have different densities, allowing for differentiation in images.
Further Considerations and Potential Pitfalls
-
Temperature Dependence: Density is temperature-dependent. The density of a substance usually decreases with an increase in temperature due to thermal expansion. Therefore, it's crucial to specify the temperature at which the density measurement was taken for accurate comparisons.
-
Pressure Dependence: Density can also be affected by pressure, especially in gases. Higher pressures generally lead to higher densities. For precise measurements, pressure needs to be controlled and recorded.
-
Units Consistency: Always ensure consistency in units throughout your calculations. Mixing units can lead to significant errors. Convert all values to the desired units before performing calculations.
Frequently Asked Questions (FAQ)
Q1: Why is the conversion factor 1000?
A1: The conversion factor arises from the relationship between grams and kilograms (1000 g = 1 kg) and milliliters and cubic meters (1,000,000 mL = 1 m³). The combined effect of these conversions results in a multiplication factor of 1000.
Q2: Can I convert kg/m³ to g/mL?
A2: Yes, you can reverse the process. To convert from kg/m³ to g/mL, divide the density in kg/m³ by 1000.
Q3: What are some common instruments used to measure density?
A3: Common instruments include pycnometers, hydrometers, and density meters which utilize various principles like buoyancy or oscillation frequency to determine density.
Conclusion
Converting density from g/mL to kg/m³ is a straightforward process that involves understanding the relationship between the metric units of mass and volume. By employing the conversion factor of 1000, one can easily transform density values between these commonly used units. Accurate density measurements are critical in diverse fields, and understanding this conversion is fundamental for any scientist, engineer, or student working with materials and their properties. Remember to always account for temperature and pressure effects to ensure the accuracy of your density determinations and maintain unit consistency for reliable results. Mastering this conversion will enhance your ability to work confidently with density values in various contexts.
Latest Posts
Latest Posts
-
Descriptive Words Beginning With X
Sep 18, 2025
-
Words That Rhyme With Air
Sep 18, 2025
-
How Do You Calculate Hdi
Sep 18, 2025
-
Words That Rhyme With Perfect
Sep 18, 2025
-
Australian Dollar To Botswana Pula
Sep 18, 2025
Related Post
Thank you for visiting our website which covers about G Ml To Kg M3 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.